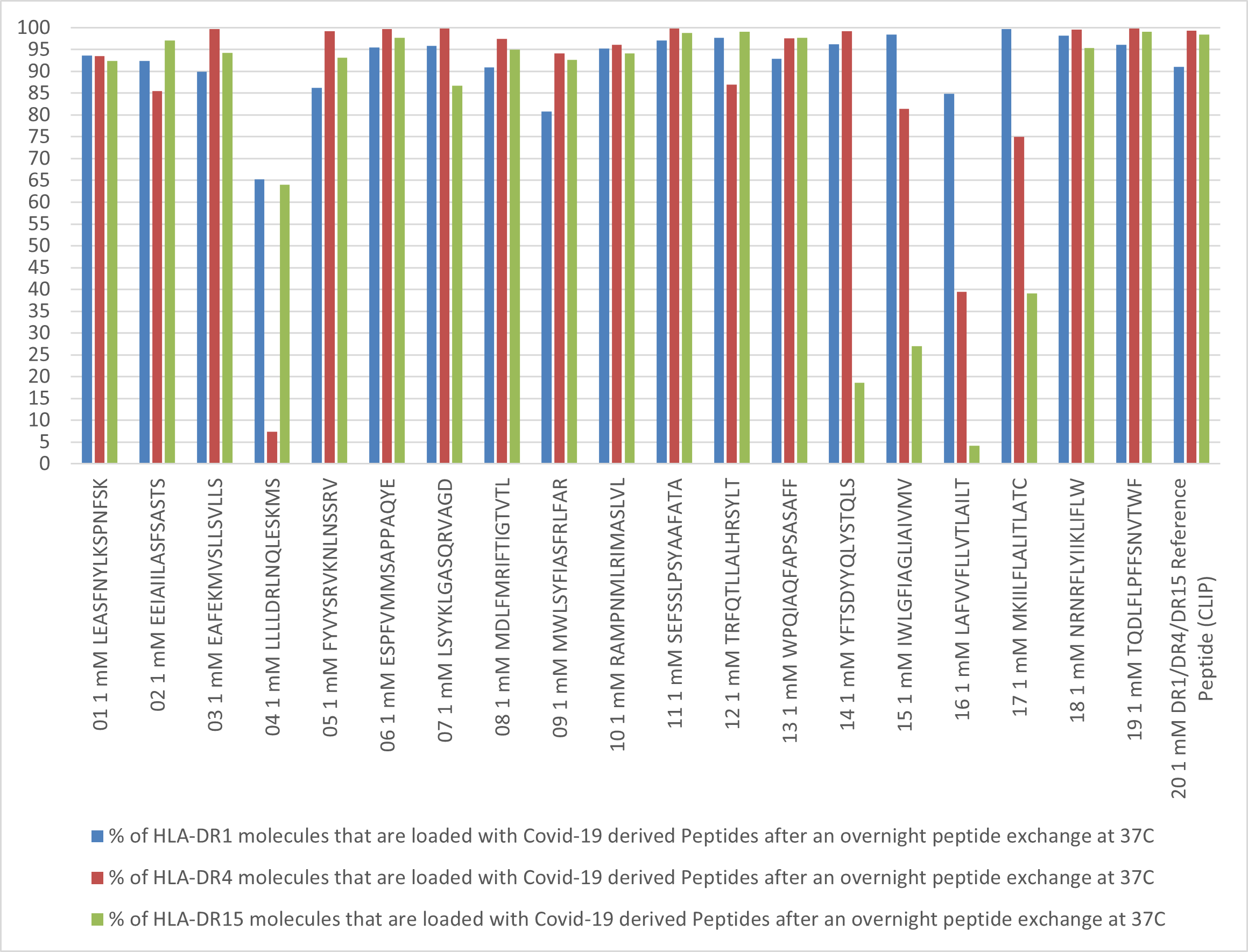
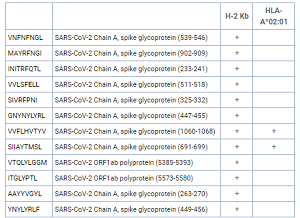
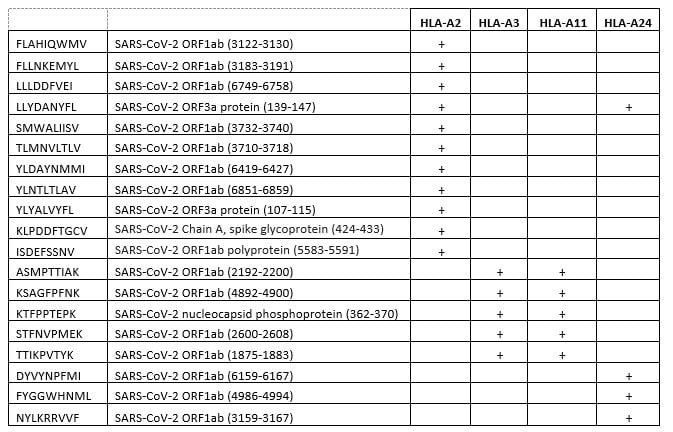
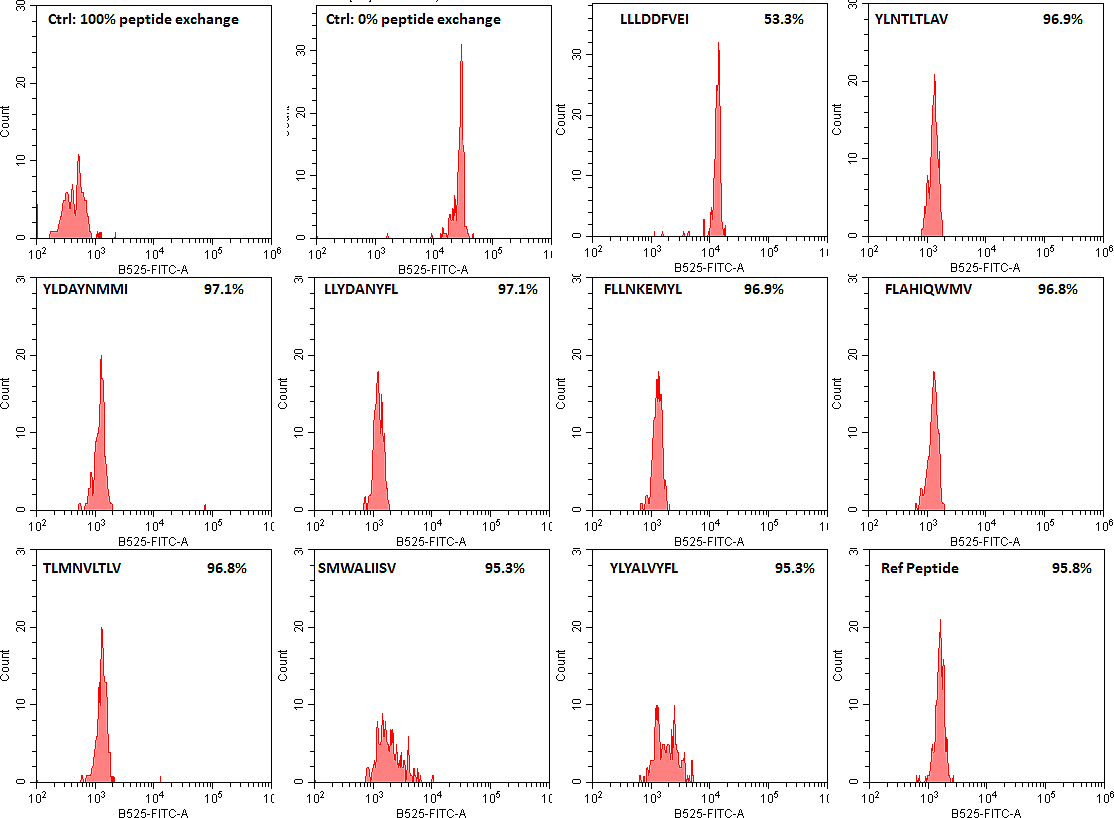
Please Note: MBL International will be shutting down its operations effective December 31, 2024. Distribution of MBL products in the United States will be transferred to Cosmo Bio US on January 1st while European Distributors will remain unchanged. For any US inquiries regarding orders or support during this transition, reach out to Cosmo Bio: https://www.cosmobiousa.com/. For Non-US inquiries reach out to MBL in Japan: https://www.mblbio.com/.