Published by Yuri Poluektov on
Please Note: MBL International will be shutting down its operations effective December 31, 2024. Distribution of MBL products in the United States will be transferred to Cosmo Bio US on January 1st while European Distributors will remain unchanged. For any US inquiries regarding orders or support during this transition, reach out to Cosmo Bio: https://www.cosmobiousa.com/. For Non-US inquiries reach out to MBL in Japan: https://www.mblbio.com/.
Published by Yuri Poluektov on Apr 8, 2020 1:00:00 PM

The use of MHC tetramers for the detection of antigen specific T cells has been a well-established technique since it originally gained prominence in the mid-1990s. Since then, the sophistication of MHC tetramer and multimer design as well as its sensitivity in detecting target T cells has been steadily improving. Most applications of MHC Tetramers involve the use of Flow Cytometry to enumerate, purify or sort specific T cells, but in some cases, it is possible to use tetramers to visualize entire immunological processes taking place inside a whole body.
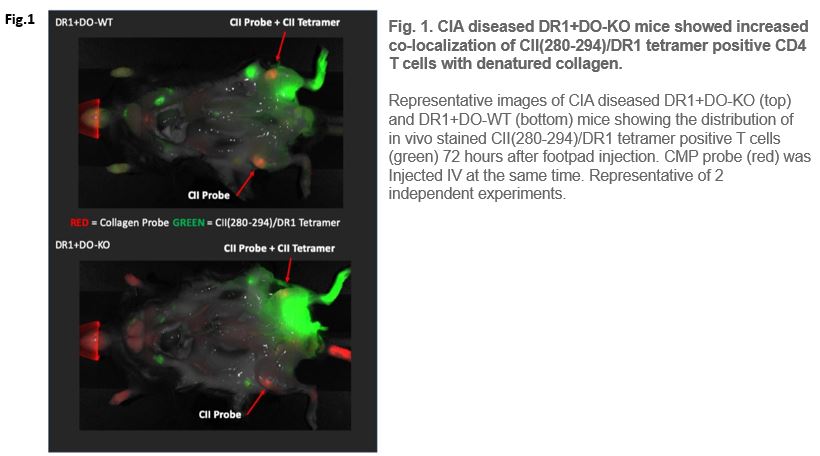
We want to bring to your attention a recent paper by Welsh and Song et. al. (Plos Biology Feb, 2020)1. In their study, while constantly using MHC Class II tetramers to detect and enumerate pathogenic T cells, responsible for the progression of Rheumatoid Arthritis (RA) as well as Experimental Autoimmune Encephalomyelitis (EAE) induced in mice models, the group performed a number of imaging trials. This imaging procedure involved a modified in vivo NIRF whole-body imaging technique described in the paper. The group was successful in identifying a co-localized population of Collagen peptide specific T-cells and a molecular probe specific for denatured Collagen protein molecules using an IRDye800CW-conjugated CII(280–294) peptide loaded HLA-DR1 tetramer.
Fig 1. CIA diseased DR1+DO-KO mice showed increased co-localization of CII(280-294)/DR1 tetramer positive CD4 T cells with denatured collagen.
Fig 1.
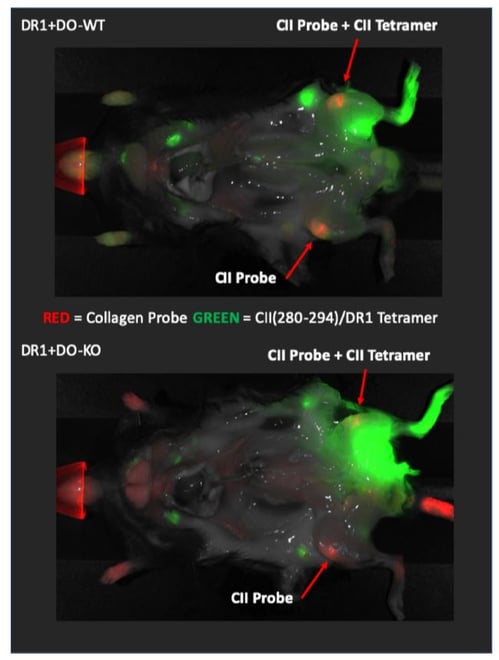
Representative images of CIA diseased DR1+DO-KO (top) and DR1+DO-WT (bottom) mice showing the distribution of in vivo stained CII(280-294)/DR1 tetramer positive T cells (green) 72 hours after footpad injection. CMP probe (red) was injected IV at the same time. Representative of 2 independent experiments.
What makes this result even more significant is that the MHC Class II tetramers are notorious for being weak binders that stain cells poorly. But this group was able to overcome this limitation and detect antigen specific T cells in vivo, presenting another application for the use of MHC tetramers in the study of immune processes. If combined with our Quickswitch™ tetramers, this procedure would be able to quickly generate an MHC tetramer with any desired peptide, without significant use of time and resources. This could potentially allow for quick screening of specific T cell populations in vivo and perhaps even the detection of multiple antigen-specific T cell populations at once.
1. Welsh and Song et. al Plos Biology 2 (https://doi.org/10.1371/journal.pbio.3000590)