Published by Beata Boczkowska, Ph.D. on Jul 13, 2023 12:05:43 PM
The HLA (human leukocyte antigen) system, also known as the human MHC (major histocompatibility complex), was named after its discovery with alloantibodies against leukocytes. The human MHC is located on the short arm of chromosome 6 (6p21) and spans 3,600 kilobases of DNA. It is divided into three regions, with HLA being one of the most diverse genetic systems in humans (1).
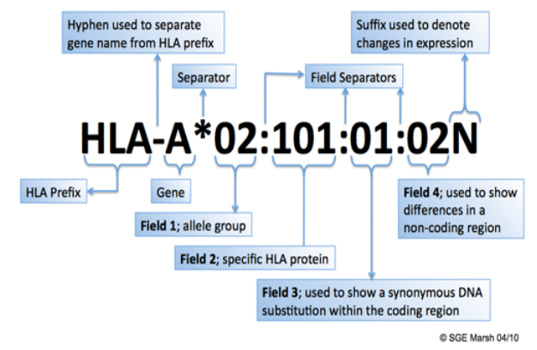
HLA allele nomenclature is assigned using a very specific system (3). This nomenclature allows for medical professionals and researchers to easily identify and/or group individuals for organ transplant matches, immunotherapy, etc. The nomenclature begins very general and becomes very specific as more information is added.
The MHC Class I region includes the HLA-A, HLA-B, and HLA-C genes responsible for encoding the heavy chains of class I molecules. The surface of nearly every nucleated cell displays the presence of HLA class I molecules, whereas class II molecules are exclusive to B lymphocytes, antigen-presenting cells (monocytes, macrophages, and dendritic cells), and activated T lymphocytes.
The MHC Class II region is comprised of several sub-regions, each housing A and B genes that encode a and P chains, respectively. The DR gene family is made up of a single DRA gene and up to nine DRB genes (DRBl to DRB9). The DRA gene encodes an invariable a chain that binds various P chains which are coded by the DRE genes. HLA-DR antigen specificities (DRl to DR18) are determined by the polymorphic DRPl chains encoded by DRBl alleles. Certain DRBl alleles contain specifically linked DRB3, DRB4, or DRB5 loci in their HLA haplotypes. The DP and DQ families each have one expressed gene for a and P chains and additional unexpressed pseudogenes. The DQAl and DQBl gene products associate to form DQ molecules, and the DPAl and DPBl products form DP molecules (1).
|
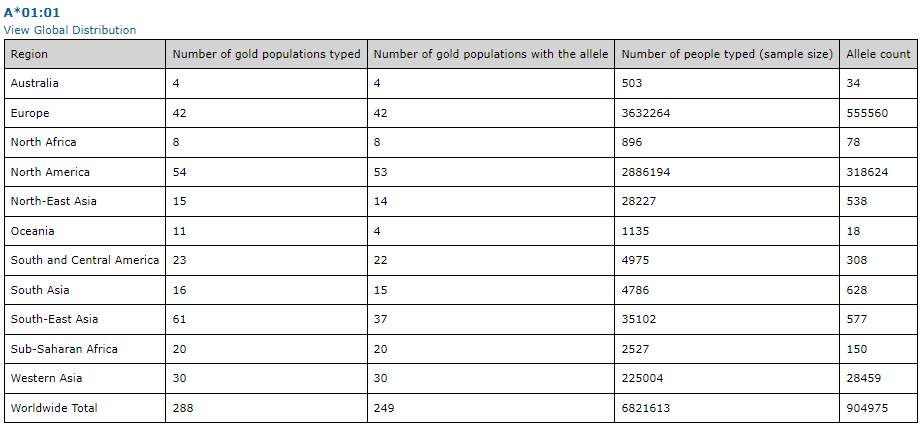
The frequency and distribution of HLA antigens vary significantly among diverse ethnic groups, suggesting that the HLA polymorphism has evolved under unique selective pressures in different geographic regions (2). This could be attributed to the role of HLA molecules in presenting prevalent infectious agents in different parts of the world (2). Databases, such as http://www.allelefrequencies.net, can be used to gather information for specific HLA information for classical or non-classical allele frequencies, as seen in the example below for HLA-A*01:01.
In solid organ transplantation, to avoid allograft rejection, accurate lymphocyte crossmatching is crucial. HLA alloimmunization can lead to complications in transfusion therapy. As transplantation antigens, the HLA system holds significant clinical importance. The MHC is responsible for identifying and rejecting foreign organs, and it encodes highly diverse cell surface molecules (1). Similarly, autoimmune diseases including Rheumatoid Arthritis (RA) show a strong association with HLA DRB1*01:01 and HLA-DRB1*04:01. However, therapeutics contain DR1 and DR4 restricted T cell epitopes, which in the RA population contribute to a high rate of reported (1). Additional, specific diseases, like Type 1 Diabetes and Multiple Sclerosis are associated with certain HLA types. Hence, this knowledge is important in most clinical trials and can greatly impact a patient's immune response to novel therapeutics (1). These examples illustrate the case for understanding your patient population in terms of HLA type, and thus knowing at an early stage how your biologic may perform in different populations.
Furthermore, a discovery by Zinkernagel and Dougherty (2) showed that T lymphocytes require identical MHC molecules as the antigen-presenting cells to stimulate an immune response. The process of peptides binding to MHC molecules and being recognized by the T-cell receptor on the cell surface is known as MHC restriction. A limited number of amino acid residues located in the peptide-binding pockets determines the peptide-binding specificities of MHC molecules as indicated in figure below.
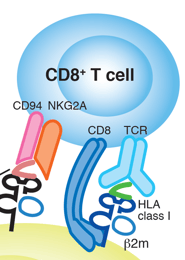
Different MHC molecules exhibit unique amino acid residue patterns in the bound peptide sequences, with conserved amino acid residues acting as anchoring points for peptides in the peptide-binding groove. The peptides that bind to class I or class II molecules have distinct natures and sources. Class I-restricted T cells can recognize endogenous antigens that are synthesized within the target cells, such as cellular, transformed, or virus-induced proteins. On the other hand, class II-restricted T cells recognize antigens that are derived exogenously from extracellular proteins. The class I or class II molecule-peptide complex on the cell surface is recognized by T-cell receptors, with class I recognized by CD8+ T lymphocytes and class II recognized by CD4+ T lymphocytes (2).
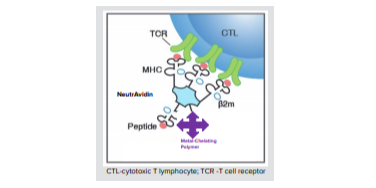
T cells play a key role in the immune response to both foreign and self-peptide antigens, which they recognize in a complex with MHC molecules. Since T cells display a low affinity for their MHC counterparts, it was historically problematic to label and analyze them effectively. This problem was solved by generating MHC multimers platform for a more stable bond between corresponding T cells. The MHC tetramer technology is based on the ability of MHC-peptide complexes to recognize the antigen-specific T cells at a single cell level. Researchers pair the MHC multimer allele they are using with the same allele as the PBMC sample. This breakthrough technology enables researchers to precisely measure targeted T-cell responses in infectious diseases, cancer, and autoimmune diseases.
MBL International provides high quality MHC Tetramers for targeted detection of specific MHC/peptide complexes. MHC/peptide monomers are biotinylated and tetramerized with streptavidin to maintain stable binding to multiple TCR, enabling MHC/peptide tetramers to be used as detection tools.
MBL International offers MHC tetramers for various Class I Human, Mouse, Rhesus Macaque, Mauritian Cyno, Chicken and Human-Mouse chimera alleles. Also offered are various Class II Human and Mouse alleles, as well as CD1d and MR1 tetramers. Phycoerythrin (PE), allophycocyanin (APC), or Brilliant Violet™ 421 (BV421) fluorophores are available for detection of antigen-specific T cells by flow cytometry or fluorescence microscopy using our MHC multimers.
References
- Lewin, H. A., G. C. Russell, and E. J. Glass. "Genomic Organization of the MHC: Structure, Origin, and Function." Immunol. Rev 167 (1999): 145-158.
- Flajnik, Martin F., and Masanori Kasahara. "Comparative genomics of the MHC: glimpses into the evolution of the adaptive immune system." Immunity 15.3 (2001): 351-362.
- SGE Marsh, J Trowsdale et al. "Nomenclature for factors of the HLA system, 2010."Tissue Antigens 2010 75:291-455