Published by Cheryl A. Guyre, Ph.D. on Mar 20, 2015 10:48:00 AM
While being a Control Freak may not be the best choice for living in spiritual harmony, it is a downright asset in flow cytometry research! Controls not only help you set up an experiment to get a clear or “true” answer, they can also help you troubleshoot what may have gone wrong when your data just doesn’t look quite right, so that your next attempt will turn out better. In flow cytometry, controls are critical to help determine “real” events from artifacts. So, what should you use as controls in tetramer experiments?
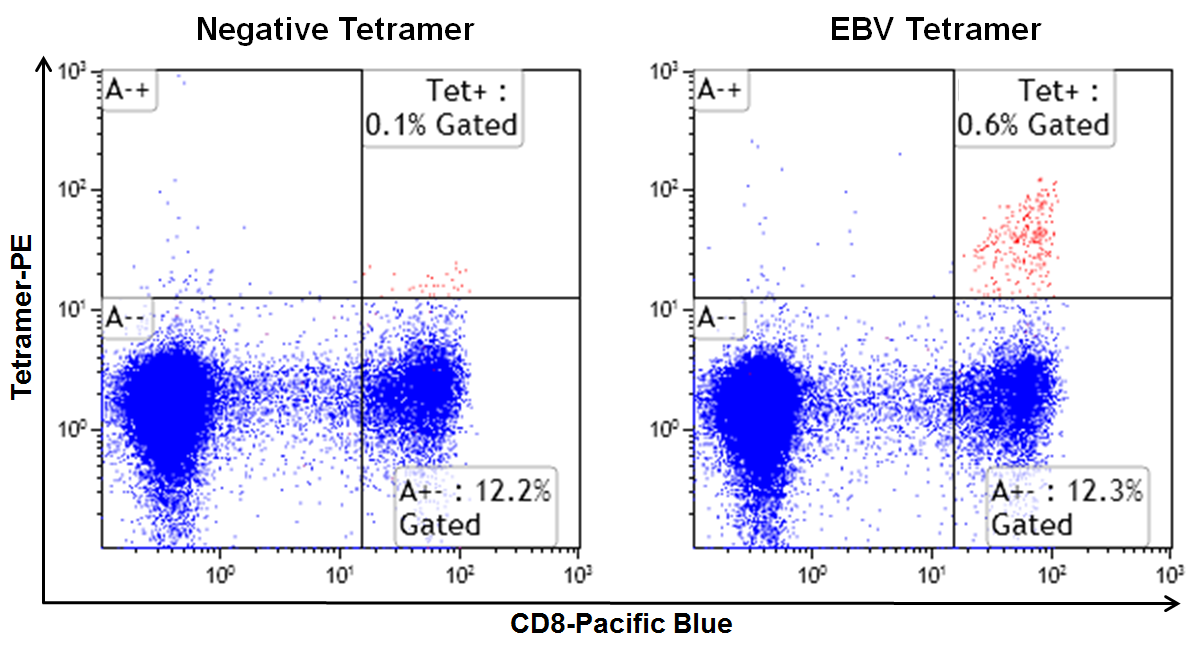
First let’s talk about negative controls. These are important in helping you set the region or gate around antigen non-specific T cells, so that you will know where to look for positive (i.e. antigen-specific) T cells. For example, in a dot plot displaying CD8 (x-axis) and class I tetramer (y-axis), a quadrant gate would be drawn based on the sample stained with a negative tetramer, ideally. The upper right quadrant (CD8+Tetramer+) is where the antigen-specific T cells events will appear when stained with the same panel containing the antigen-specific tetramer. An appropriate negative control for a particular tetramer will have four basic elements:
- Same fluorochrome
- Same MHC allele
- Peptide known to have no reactivity in the sample or in the system studied
- In the context of the exact flow antibody panel, with the identical staining conditions and acquisition parameters/settings.
Often stocked tetramer products or customs with specificities unrelated to the experiment may be appropriate, but ultimately, it is up to the investigator to select the control most appropriate for his or her particular model or experiment. Here are some tips:
Options for negative controls for human tetramer experiments:
- For HLA-A*02:01 alleles, MBLI offers a “Negative Tetramer” in both PE (TB-0029-1) and APC (TB-0029-2). This tetramer is prepared with the HLA-A*02:01 allele and a proprietary peptide whose sequence does not occur in nature. No, I cannot tell you the sequence, as it is proprietary (and I couldn’t tell you anyway, as the R&D folks won’t even tell me what it is!). Class I tetramers with “no peptide” cannot be made, as the empty class I molecule is unstable.
- While some researchers choose the Negative Tetramer to control for different HLA class I alleles, it is really better to select a matched allele. These may be in the form of another stocked product of that allele having a specificity known to be unreactive for the donor, or a custom tetramer whose peptide sequence you choose. For Class II alleles, tetramers made using the human CLIP peptide or a non-specific peptide can serve as negative controls.
- Another type of negative control is a negative donor. One researcher studying HLA-A*02:01-positive autoimmune patients had the clever solution of using the patient’s healthy partner or family member as a sort of “paired” negative control. The Negative Tetramer plus the specific tetramer of interest was used both on the patient and the HLA-A*02:01-positive, but non-autoimmune, loved one.
Options for negative controls for mouse tetramer experiments:
In mouse tetramer experiments, there are generally two options for selecting a negative control that will allow proper region/gate placement:
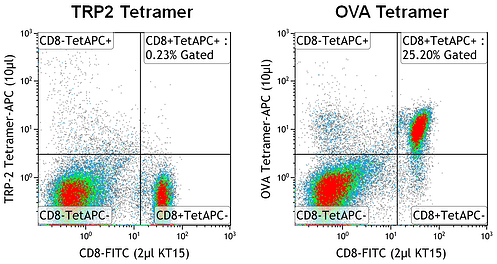
- A tetramer of the same allele and fluorochrome as the specific tetramer, built with a non-specific peptide or a specificity known to be unreactive in the mouse model of interest may be appropriate. For example, H-2Kb tetramers specific for either β-gal or TRP2 have been used as negative controls in experiments using splenocytes from OT-I mice transgenic for the T cell receptor that binds specifically to H-2Kb OVA (SIINFEKL). For proper gate placement, the full panel of antibodies used for the experimental tetramer stain must be included in the control tetramer stain to account for fluorescent spillover contributed by fluorochromes from other channels of the flow cytometer.
- In experiments involving immunized or treated mice, often cells from a naïve mouse, stained with the same tetramer/antibody panel as the experimental mouse cells, is an appropriate negative control.

