The Principle and Method of Chromatography
The principle and method
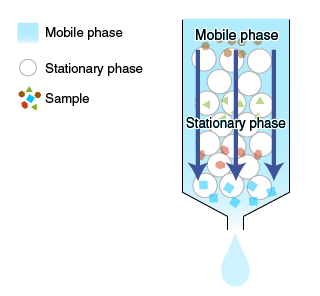
Chromatography is a technique for separating the components of a mixture based on differences in their interactions with surrounding substances.
When a solute in a solvent (or a mobile phase) is passed through or around the outside of a matrix (or a stationary phase), interactions occur between the solute and the stationary phase. Solutes with different properties are separated based on differences in these interactions.
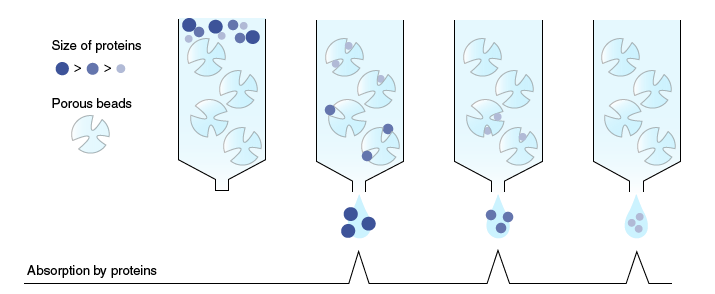
Gel-filtration chromatography
In gel-filtration chromatography (also known as molecular sieve chromatography), proteins are separated according to their molecular weight. When a sample is passed through a column packed with a matrix of porous beads, low molecular weight proteins flow through and around the beads in the direction of solvent flow, and high molecular weight proteins flow around the beads without interacting with the matrix material. Consequently, progression through the column is slower for smaller proteins than for larger ones. As a result, proteins are separated according to their size.
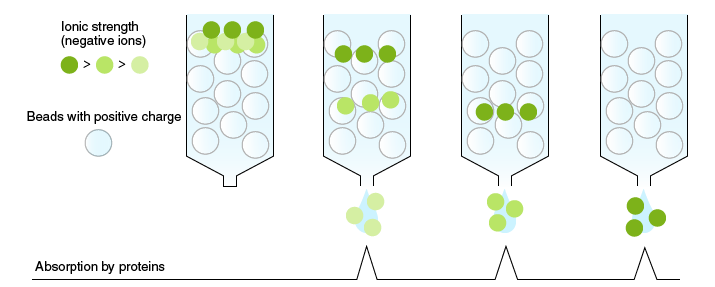
Ion-exchange chromatography
In ion-exchange chromatography, proteins are separated according to differences in their charge. Proteins have a net charge, which is the basis for protein interaction with ion-exchange media. Basic proteins with a positive charge bind ionically to a negatively charged matrix (cation exchanger), and acidic proteins with a negative charge bind ionically to a positively charged matrix (anion exchanger). Samples are applied to a column packed with an ion exchange resin. The elution of proteins in these samples from the column depends on gradually increasing the salt concentration of the mobile phase, which weakens the ionic interactions and facilitates their downward movement. Consequently, proteins that interact weakly with the resin elute first and the elution order and resolution of the separated proteins depends on their charge.
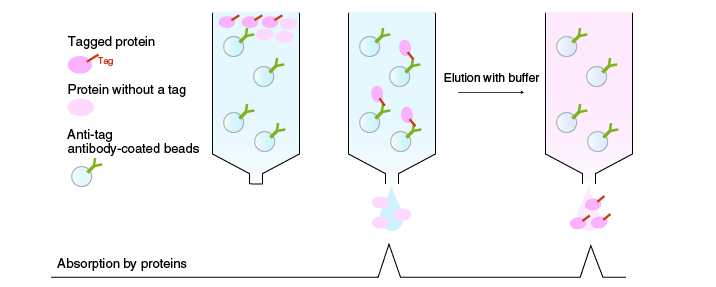
Affinity chromatography
In affinity chromatography, proteins are separated according to differences in their binding affinity. Enzymes, receptors, and antibodies have high binding affinity for specific ligands. When a mixture of proteins is passed through a column packed with a ligand-coupled matrix material, only proteins that bind to the ligand will be retained. The target protein is eluted by a solution containing a solute that competes for the binding site of the target protein, i.e., the ligand (or its analogue).
A common example is purification of a recombinant protein. A defined peptide sequence called an epitope tag is often added to a recombinant protein. Highly purified recombinant protein is obtained by affinity chromatography using epitope-specific antibodies bound to a matrix. Other examples include purification of proteins fused with a fluorescent protein, carbohydrate binding protein, or metal binding protein.
Previous page: The principle and method of SDS-polyacrylamide gel electrophoresis (SDS-PAGE)
Related Links
Antibody
Antibody basics
Antibodies as a research tool
- How to generate antibodies
- How to select antibodies
- Labeled antibodies
- How to label antibodies
- Main causes of non-specific reactions
- How to reduce non-specific reactions
- Tags and Tag antibodies
Qualitative and quantitative measurements of proteins using antibodies
- Western blotting (WB)
- Enzyme-linked Immunosorbent assay (ELISA)
- Immunoprecipitation (IP)
- Co-immunoprecipitation (Co-IP)