Published by Bindi M. Doshi, PhD on Jun 13, 2019 1:00:00 PM
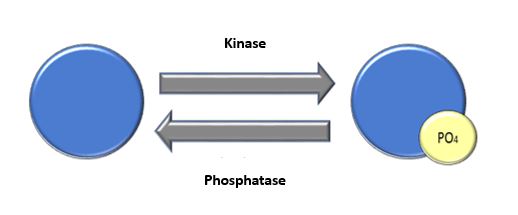
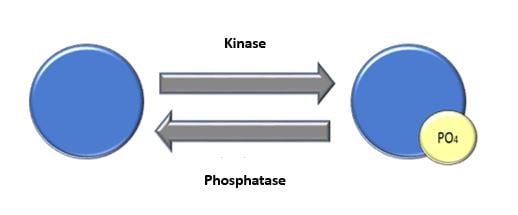
Protein phosphorylation is quintessential for specific pathways to function. This function can be influenced by internal or external factors. It is a reversible action with the involvement of kinases and phosphatases1. This activity is crucial in the cell cycle, apoptosis, and signal transduction pathways. If a protein is phosphorylated or dephosphorylated when it shouldn’t be, there can be severe disruptions to the cellular pathway. At times, the phosphorylation state can be the cause of a disease. This has been found in degenerative diseases, cancers, and various pathways involving the immune system2. For instance, kinase inhibitors have been successful in cancer treatments.
Enzymes control phosphorylation. Kinases function to add phosphate groups to proteins and phosphatases function to remove phosphates. Typically, phosphorylation occurs on serine, threonine, or tyrosine residues. This process can be very quick or it can take many hours. It results in a conformational change which either activates or inactivates protein activity.
Phosphorylation in disease states is widely studied. Why does phosphorylation go awry? What is the consequence of this? Is there a way to make corrections? For instance, Alzheimer’s disease has been found to be influenced by the amyloidogenic peptide, Aβ. Protein phosphorylation affects cellular pathways. In fact, Aβ inhibited phosphatases, leading to changes in APP and TAU proteins. When additional proteins were analyzed for changes directly corresponding to Aβ and phosphatase inhibition, a pool of potential biomarkers were discovered, and these can be further analyzed to understand Alzheimer’s disease and possible treatments3.
As we further our understanding of phosphorylation and protein regulation, we can develop drugs where there are kinase inhibitors or activators. There are kinase inhibitors that are used for treatment of chronic inflammatory diseases4. Research and understanding of GSK3 kinase led to the advancement of drug development for diabetes treatment5.
Understanding signaling cascade is crucial in understanding cancer and finding potential treatments. Complicating cancer research is that many different signaling pathways and proteins seem to be implicated in disease progression. MAPK has been found to be very important in cancer growth while Bcl-2 has a role in apoptosis. There are drugs for tyrosine kinases that show promise as anti-cancer drugs, showing how important protein phosphorylation is in cancer treatments6.
There are a few different ways to detect if your protein of interest is phosphorylated or not. The simplest way is to use an antibody directed to the phosphorylation site. You can look at specific phosphorylation sites or look at total phosphorylation if your protein has more than one phosphorylation site. Western blots are used to monitor protein presence or expression levels. There are also kits available that can help semi-quantify phosphorylation levels in your cells. These kits can look at kinase activity or come as an ELISA kit.
Click below to see the various antibodies and kits MBLI provides for researching the phosphorylation of your protein of interest.
- Ardito, F., Giuliani, M., Perrone, D., Troiano, G., & Muzio, L. L. (2017). The crucial role of protein phosphorylation in cell signaling and its use as targeted therapy (Review). International Journal of Molecular Medicine,40(2), 271-280. doi:10.3892/ijmm.2017.3036 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5500920/
- Ferguson, F. M., & Gray, N. S. (2018). Kinase inhibitors: The road ahead. Nature Reviews Drug Discovery,17(5), 353-377. doi:10.1038/nrd.2018.21 https://www.nature.com/articles/nrd.2018.21
- Henriques, A. G., Müller, T., Oliveira, J. M., Cova, M., Cristóvão B. Da Cruz E Silva, & Odete A. B. Da Cruz E Silva. (2016). Altered protein phosphorylation as a resource for potential AD biomarkers. Scientific Reports,6(1). doi:10.1038/srep30319 https://www.nature.com/articles/srep30319
- Rokosz, L. L., Beasley, J. R., Carroll, C. D., Lin, T., Zhao, J., Appell, K. C., & Webb, M. L. (2008). Kinase inhibitors as drugs for chronic inflammatory and immunological diseases: Progress and challenges. Expert Opinion on Therapeutic Targets,12(7), 883-903. doi:10.1517/14728222.12.7.883 https://www.tandfonline.com/doi/abs/10.1517/14728222.12.7.883?journalCode=iett20
- Cohen, P. (2012). Protein Phosphorylation in Human Health. doi:10.5772/2944 https://febs.onlinelibrary.wiley.com/doi/full/10.1046/j.0014-2956.2001.02473.x?sid=nlm%3Apubmed
- Singh, V., Ram, M., Kumar, R., Prasad, R., Roy, B. K., & Singh, K. K. (2017). Phosphorylation: Implications in Cancer. The Protein Journal,36(1), 1-6. doi:10.1007/s10930-017-9696-z https://link.springer.com/article/10.1007%2Fs10930-017-9696-z
