Published by Beata Boczkowska, Ph.D. on Mar 10, 2023 10:45:21 AM
Innate Immunity is a nonspecific host defense mechanism against foreign antigens. It is the first line of defense in a host and occurs immediately or within hours after exposure. This type of immunity is one that a host is born with, in turn it is known as natural or innate immunity.
The most effective mechanisms of innate immunity are 1) anatomical barriers includes skin and mucous membranes, 2) intact normal flora, 3) soluble proteins which includes complement, acute phase proteins and interferons, and 4) ability to induce inflammatory and phagocytic response.
The innate immune response has the ability to distinguish molecules shared by groups of related antigens but does not recognize every conceivable antigen. The innate immune response is not activated by normal flora microbes essential for the survival of a host. However, unique microbial molecules referred to as pathogen-associated molecular patterns or PAMPs which include LPS from the Gram-negative cell wall, peptidoglycan and lipotechoic acids from the Gram-positive cell wall, shared sugar mannose in microbial glycolipids and glycoprotein, bacterial and viral unmethylated CpG DNA double-stranded and single-stranded RNA from viruses, and glucans from fungal cell walls are all identified as foreign antigens. Other than foreign antigens, the innate immune response can identify stressed, injured, infected, or transformed human cells which lead to the activation of multiple pathways to signal danger to the host and take subsequent immediate action.
The detection of PAMPS occurs by multiple receptors on various cell types. The Toll like receptors (TLR) are the most prevalent PAMP receptors on numerous immune cells, including macrophages, dendritic cells (DCs), B cells, along with nonimmune cells such as fibroblasts and epithelial cells. TLRs recognize different PAMP depending whether the TLRs are expressed on the surface of the cell (TLR 4,5,6) or within the intracellular compartments (TLR 7,8,9). An example of a common activator of surface TLR is by Lipopolysaccharides (LPS) the outer membrane components of gram-negative bacteria. Additionally, released LPS binds with LPS binding protein (LBP) an acute phase protein in the blood stream. The LPS complexes with LBP and binds to a glycosylphosphatidylinositol (GPI) CD14 on the cell membrane. The PGPI-linked protein is present on the surface of mononuclear phagocytes and as a soluble protein in the blood. In human serum, the soluble form of CD14 (sCD14), which lacks the GPI anchor, is involved in LPS-induced cell activation. The presence of sCD14 was shown to be present in plasma with infectious diseases. A different example of PAMP engagement is by the use of Mannose receptors and binding of sugar mannose in microbial glycolipids and glycoprotein its. The soluble mannose receptor(s), MR is cleaved by matrix metalloproteinase on the cell membrane and secreted into the peripheral serum. Consequently elevated levels of sMR may signal serious illness such as sepsis, pneumonia, and acute hepatitis.
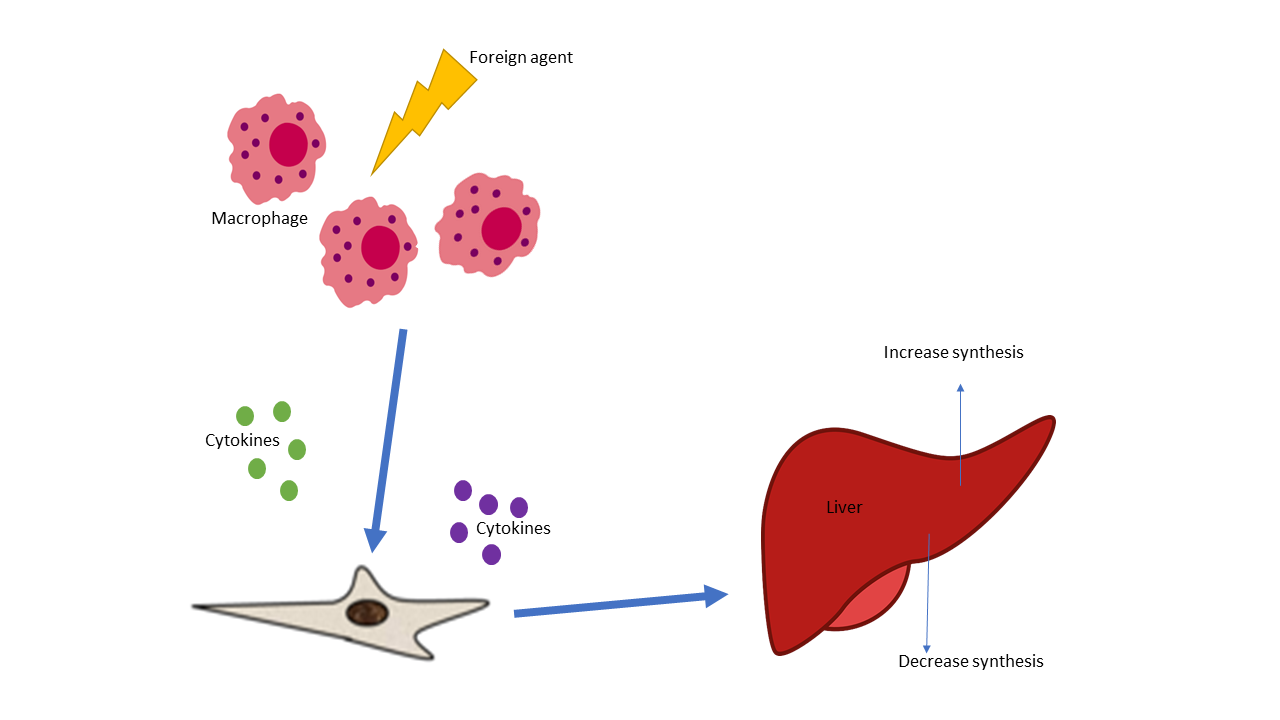
Different Pathogen Recognition Receptors (PPR), like TLR and MR are essential to the host innate immunity and defense against foreign antigens. When PRRs bind PAMPs like LPS a series of reactions occur in the host. The acute phase response of innate immunity defense is activated and involves the production of pro-inflammatory mediators and acute phase proteins into the bloodstream or injured tissue. Thus contact with bacterial or fungal components triggers acute phase response and inflammatory cells to increase proteins like TREM-1 which is widely expressed on monocytic cells and amplifies innate immune response, making TREM 1 levels an indicator of sepsis. The acute-phase (AP) proteins occur in inflammatory conditions and are detected rapidly in plasma concentration in response to chemical and cellular signaling due to injury or foreign invasion. Some acute phase proteins increase, referred to as positive acute-phase proteins while other AP proteins decreases (negative acute-phase proteins) (Table). Acute phase proteins can increase transiently (C-reactive protein) while others have a more sustained elevation (Haptoglobin). Human Serum amyloid P component (SAP) is not an acute phase reactant following acute stimuli, in contrast to mouse SAP and human CRP. SAP is a normal plasma constituent that is present in cerebrospinal fluid, in the pathognomonic lesions of Alzheimer's disease, cerebrovascular and intracerebral A & beta; amyloid plaques and neurofibrillary tangles. 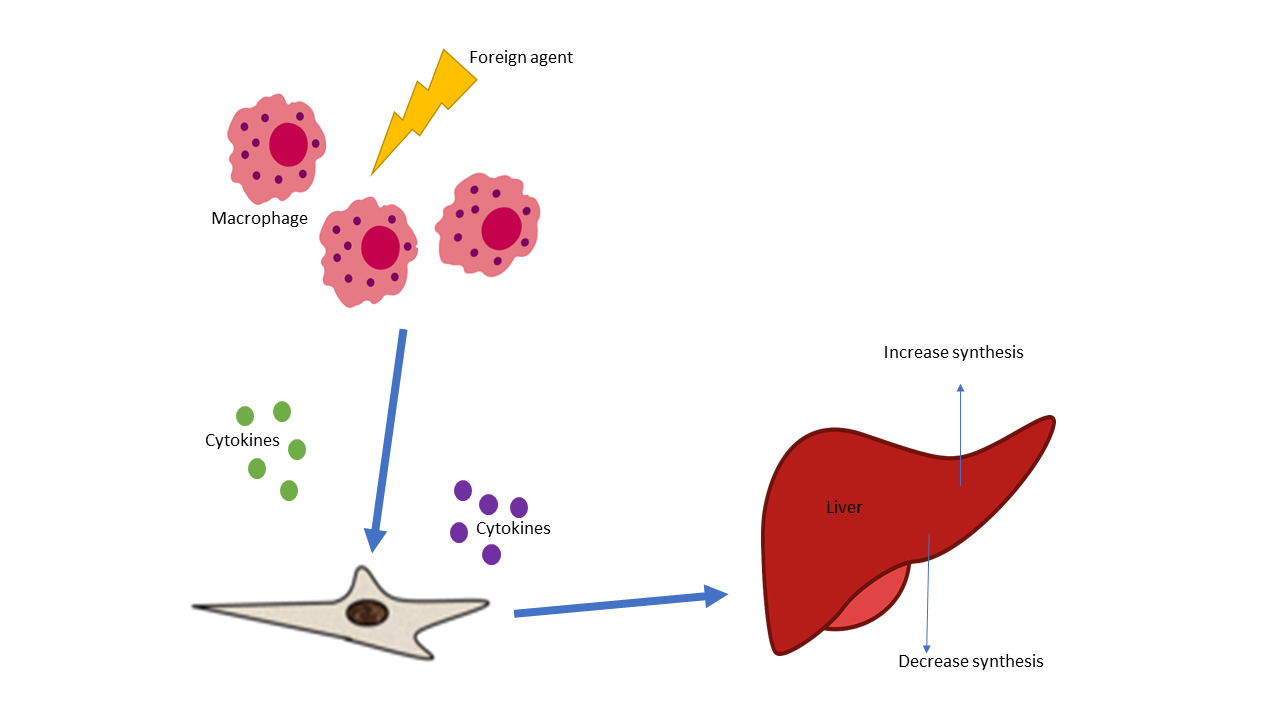
|
Positive Acute Phase Proteins |
Negative Acute Phase Proteins |
|
C-reactive protein (CRP) |
Albumin |
|
Complement (C3, C4) |
Transferrin |
|
Fibrinogen |
Retinal-binding protein |
|
Serum Amyloid |
Adiponectin |
|
Haptoglobin |
|
|
Protein S |
|
|
Haptoglobin |
|
There are multiple components of the acute phase of innate responses which can occur together or not all leading to various illness or disorders. The acute phase response is considered part of the innate system and plays a role in fever, leukocytosis increased cortisol decreased thyroxine, decreased iron and many others. The major acute phase protein, such as C-reactive protein, is produced by the liver in response to tissue damage. Additionally, this acute-phase protein is involved in in the activation of the complement system via the classic pathway.
Acute phase proteins are an integral part of the complement pathway in turn the innate immune response. The complement system is comprised of nearly 30 proteins circulating in plasma and expressed on cellular surfaces. Once activated, different proteins act together in a particular sequence called the complement cascade. Complement (C3 and C4) are considered part of the positive acute phase response and are the generally the most evaluated complement proteins, along with total complement. Circulating C3 is synthesized in the liver and comprises 70% of the complement system, but cells in other tissues can also produce C3. C3 is an essential activating protein in the classic and alternate complement cascades. Enzymes from classical, lectin or the alternative complement pathways further degrade C3 into C3a, C3b complement components. The degradation continues down the complement cascade when C3b is converted into C3c. Consequently, elevated levels of Human C3c may indicate unrestrained complement activation and signaling an inflammatory state and autoimmune. The APP C3 is essential to multiple complements pathways whereas C4 takes part in the classic complement pathway.
The classical pathway is triggered by antigen-antibody complexes and includes participation of all complement proteins C1 through C9. The classical pathway is initiated by the binding of C1q to antigen–antibody complexes. C1q is a ligand of gC1qR and is involved in the early innate immune defense against infection. The gC1qR is presents a range of cells and a binding site for plasma. Along with its well-defined role in the complement cascade, C1qR contributes to the phagocytosis, procoagulant activity, and chemotactic activity on mast cells, eosinophils, neutrophils, and fibroblasts. Complement components like C1q, are essential soluble danger sensing proteins that are activated by both endogenous and exogenous stimuli. Still, it is the five late components of the complement system That lead to the formation of the terminal complement complex (TCC). TCC is a potent pro-inflammatory complex of the late complement components in the host defense.
The complement system contributes significantly to inflammatory response, in addition, its components are involved in thrombosis and the coagulation system. The coagulation system connects with the inflammatory response via plasma. Coagulation activates proteases such as FXII a serine protease that not only plays a role in blood coagulation and the complement system.
The central component of the organism’s defense strategy, especially in the context of innate immunity is the immediate response of the acute phase reaction. Accordingly, the inflammatory response is activated along with all the successive innate systems which may include the complement and /or coagulation to ultimately resolve of the pathological disruption.
References:
1. Shizuo Akira, Satoshi Uematsu, Osamu Takeuchi, Pathogen Recognition and Innate Immunity, Cell ,Volume 124, Issue 4,2006,Pages 783-801.
2. Gruys, E et al. “Acute phase reaction and acute phase proteins.” Journal of Zhejiang University. Science. B vol. 6,11 (2005): 1045-56. doi:10.1631/jzus.2005.B1045
3. Coen Maas, Thomas Renné; Coagulation factor XII in thrombosis and inflammation. Blood 2018; 131 (17): 1903–1909. doi: https://doi.org/10.1182/blood-2017-04-569111
