Published by Bindi M. Doshi, PhD on Mar 17, 2023 4:34:51 PM
Organoids are 3D structures made up of cells that replicate the structure and function of tissues and organs. Organoids can be derived from stem cells or tissue-specific progenitor cells. A stem cell niche is the specific microenvironment that regulates the stem cell behaviors. Many researchers try to recreate these stem cell niches in vitro. However, it is difficult to create the appropriate cell culture conditions for stem cell growth. Here we discuss current advances made to help create and maintain organoid cultures.
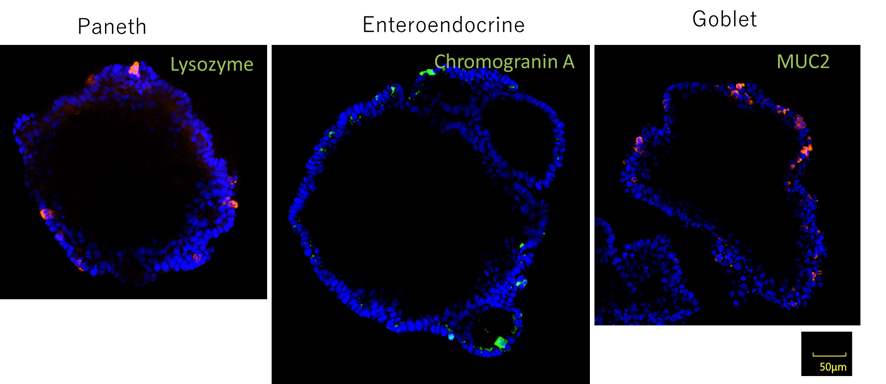
In 2009, Prof. Toshiro Sato and Hans clever achieved the in vitro expansion of the adult stem cells from mouse intestine by reconstituting stem cell niche. In brief, the stem cell is embedded in ECM gel, such as Matrigel, and is maintained and extended with the culture media containing a defined cocktail of niche factor. In this advanced 3D culture technique, mouse intestinal stem cells form self-organized clusters, named as organoids. The organoid has various differentiated functional cell types found in vivo, and also recapitulates in vivo structure, villus and crypt.
In forming organoid cultures in your lab, there are times that even the best techniques will still present with issues for organoid formation. Using a high quality and active Wnt agonist is the most effective way for success of organoid culture. Wnt3a is considered a niche factor of organoid culture.
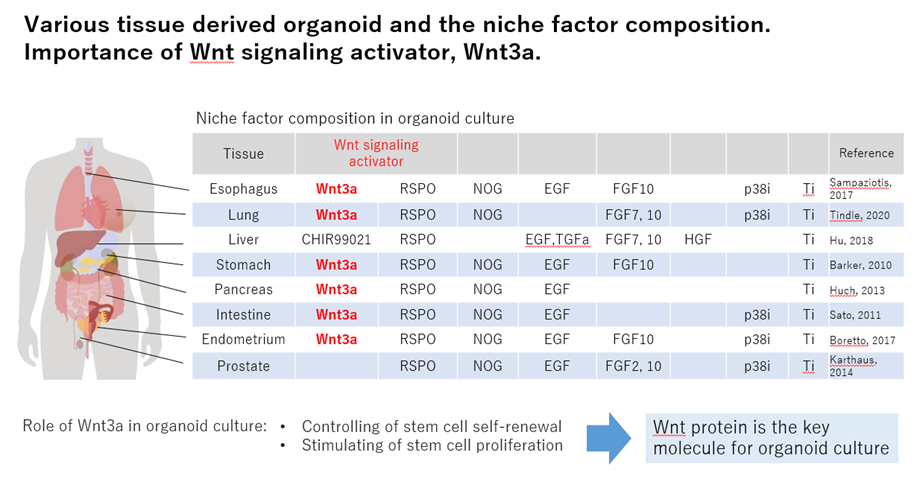
There are two options as a source of Wnt3a protein for organoid culture, one is a purified recombinant Wnt3a protein and the other is a Wnt3a-CM. Each Wnt protein has a specific problem.
In purified recombinant Wnt protein, the detergent CHAPS is used for retaining of its solubility. But, CHAPS concentrations above 0.25% show cytotoxicity. On the other hand, the self-renewal activity of Wnt3a was canceled in ES cells culture even under the culture condition of 0.02% CHAPS which is far below the cytotoxic concentration. On dilution of recombinant Wnt3a into serum-free culture medium, wnt3a proteins rapidly aggregate and lose that activity, because the detergent concentration drops below the level required to maintain Wnt solubility. Therefore, for sustaining Wnt efficacy in organoid culture, a medium change with extremely high frequency is needed.
In Wnt3a-CM, the CM from L-Wnt-3A contains 10% FSB. It is recognized that serum is essential factor for Wnt solubilization because Wnt3a protein is not secreted efficiently into culture supernatant in the absence of serum. However, serum contains unknown factors. In the case of adapting of this CM for organoid culture, 50% CM will be needed as a final concentration. It results that unknown factors which is contained in 5% serum will be carried in the organoid culture medium. Therefore, for sustaining of Wnt activity and/or efficient production of Wnt protein, development of the solubilizing method without detergent and serum had been desired.
In 2016, Dr. Mihara and Prof. Takagi identified a plasma glycoprotein, Afamin, as the serum component which is responsible for the stabilizing of Wnt proteins in the cell culture media. They also established a cell line with stable expression of mouse Wnt3a and human Afamin, and achieved its conditioned medium in serum-free condition. Purified Wnt3a:AFM complex from this conditioned medium maintained its activity after the dilution under cell culture condition, and this biological activity is higher than that of commercial recombinant Wnt3a protein. This research was ground breaking for both Wnt proteins and Organoids.
Since 2019, MBL has been selling the AFM/Wnt3a-CM and purified AFM/Wnt3a protein from this CM. By optimization of the production procedure, Wnt3a protein yield is stable in high level, about 485 ng/mL. In every production Lot, Wnt3a protein concentration of CM are checked, and the biological activity are also measured. For keeping the biological activity of our AFM/Wnt3a-CM in the organoid culture, medium must be changed every 2 or 3 days.
Specific FGFR ligands are used for some organoids, depending on those ligand requirements. When the medium refinement using FGF-2 are apply to all organoid culture, FGFR ligands will be required up to 3 molecular species, FGF-2, -7 and -10. A universal FGFR ligand will not only solve the complexity of niche cocktail, but also move forward organoid research. FGF1 is considered a universal FGFR ligand since it can bind to all FGF receptors with high affinity. With some protein modifications, our team has developed a thermal stable and universal FGFR ligand called FGF-Max.
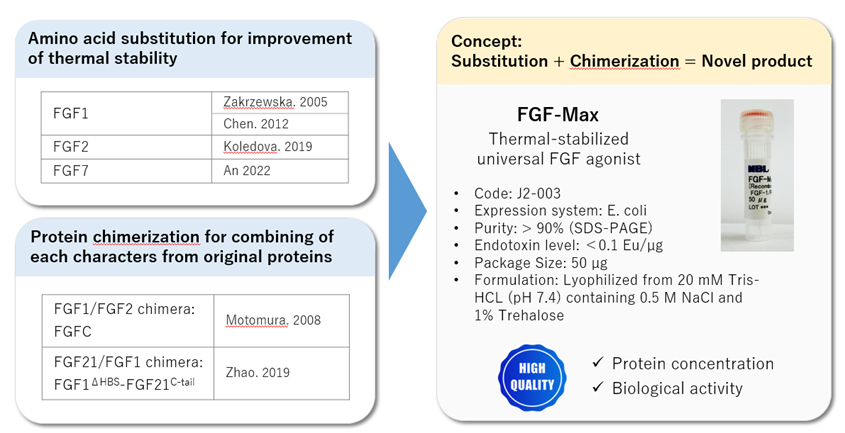
It is clear that stem cell cultures and organoid cultures require niche factors. It is not only important to have excellent technique, but also to be using high quality, high activity, and function products. Afamin/Wnt3a and FGF-Max are 2 components that have helped researchers greatly. Please contact us with any questions or guidance with your organoid cultures

