Published by M. Mamunur Rahman, PhD on Mar 27, 2019 2:00:00 PM

Organoids are self-organized three-dimensional tissue cultures that are derived from mammalian pluripotent or adult stem cells. These in-vitro-grown cell clusters are given possibilities to replicate much of the complexity of an organ. There are potentially as many types of organoids as there are different tissues and organs. To date, researchers have been able to prepare brain, kidney, lung, intestine, stomach, liver and many more are developing.
In 2009, Dr. Sato described the successful derivation of epithelial organoid cultures from single Lgr5+ stem cells (Sato et al. 2009). To maintain the organoid culture, researchers must be able to maintain the stem cells niche without feeder. Stem cells proliferation first create cystic spheroids, which then form crypt-like buddings that further develop in “mini-guts” with distinct crypt-villus compartmentalization as seen in vivo. In organoid growth, proliferating cells also express stem cell markers such as Lgr5 (Shyer et al., 2015). This phenotype can be maintained in vitro by experimentally stabilizing b-catenin, the cytoplasmic effector of Wnt signaling (Clevers and Nusse, 2012), either by supplementing the medium with Wnt3a or by generating mutant organoids expressing non-degradable b-catenin (Drost et al., 2015; Sato et al., 2011b).
Wnt signaling plays an important role in early development, maintenance (Logan et al. 2004) and regeneration of stem cells (ten Berge 2011), and in cancer formation (Zimmerman 2012). Wnt3a has been revealed to be an essential niche component for maintaining the proliferation of Lgr5-positive stem cells in various organoids such as the small intestine, large intestine, stomach, pancreas and liver. Although Wnt3a has been conventionally used for the culture of gut organoids, it is a fat-soluble protein, so it forms aggregates in serum-free medium and cannot exert its activity sufficiently. Wnt secretion from cultured cells require the presence of bovine serum in the media (Willert, 2008). As a result, Wnt-containing culture media would always have to contain ~10% serum, precluding the use of such sample in biological assays that are sensitive to the factors contained in bovine serum.
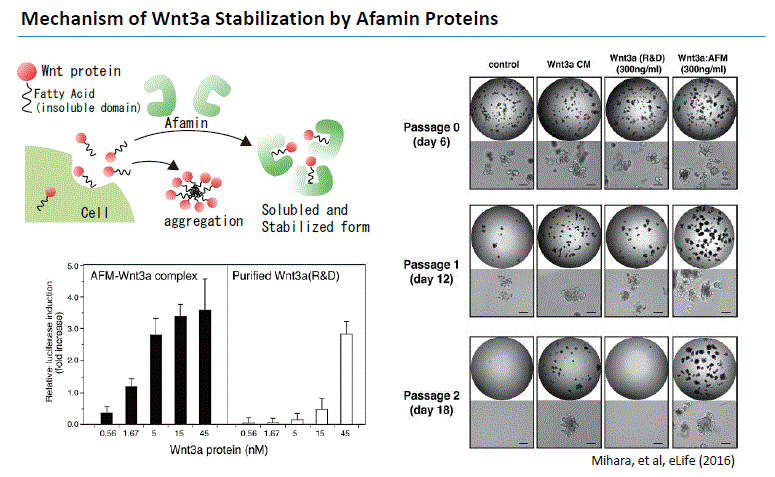
In 2016, Mihara and Sato et al. found that high Wnt3a activity can be maintained by forming a complex with Wnt3a by Afamin, which is one of the components of serum. They found that Afamin and Wnt apparently make 1:1 complex in the culture media which is highly soluble in detergent-free buffers upon isolation. Afamin-Wnt complex is biologically active indicating that Afamin can deliver Wnt ligands to its receptors on cell surface (Mihara and Sato et al. 2016). In addition, by using Afamin and Wnt3a complex for organoid culture, long-term culture of organoid becomes possible. MBL International is proud to bring this medium into the market which will help our organoid researchers. This new medium will result in optimal success for your organoid experiments.
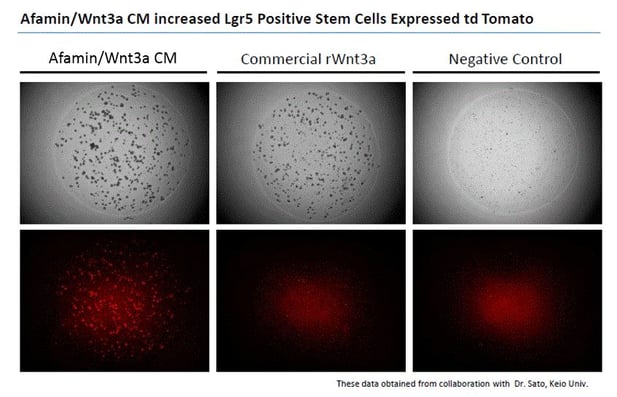
Images on the top panels show a bright field of human color organoids. Images on the bottom panels show fluorescent LGR5 positive stem cells that express td tomato regulated by Lgr5 promoter. Afamin/Wnt3a CM maintained LGR5 positive stem cell growth is seen at greater levels compared with cell growth in commercial recombinant Wnt3a (300ng/mL).
References
Sato, T., Vries, R.G., Snippert, H.J., van de Wetering, M., Barker, N., Stange, D.E., van Es, J.H., Abo, A., Kujala, P., Peters, P.J., et al. (2009). Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459, 262–265.
Shyer, A.E., Huycke, T.R., Lee, C., Mahadevan, L., and Tabin, C.J. (2015). Bending gradients: how the intestinal stem cell gets its home. Cell 161, 569–580.
Clevers, H., and Nusse, R. (2012). Wnt/beta-catenin signaling and disease. Cell 149, 1192–1205.
Drost, J., van Jaarsveld, R.H., Ponsioen, B., Zimberlin, C., van Boxtel, R., Buijs, A., Sachs, N., Overmeer, R.M., Offerhaus, G.J., Begthel, H., et al. (2015). Sequential cancer mutations in cultured human intestinal stem cells. Nature 521, 43–47.
Sato, T., van Es, J.H., Snippert, H.J., Stange, D.E., Vries, R.G., van den Born, M., Barker, N., Shroyer, N.F., van de Wetering, M., and Clevers, H. (2011b). Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature 469, 415–418.
Logan CY, Nusse R. 2004. The Wnt signaling pathway in development and disease. Annual Review of Cell and Developmental Biology 20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126
ten Berge D, Kurek D, Blauwkamp T, Koole W, Maas A, Eroglu E, Siu RK, Nusse R. 2011. Embryonic stem cells require wnt proteins to prevent differentiation to epiblast stem cells. Nature Cell Biology 13:1070–1075. doi: 10.1038/ncb2314
Zimmerman ZF, Moon RT, Chien AJ. 2012. Targeting wnt pathways in disease. Cold Spring Harbor Perspectives in Biology 4:a008086. doi: 10.1101/cshperspect.a008086
Willert KH. 2008. Isolation and application of bioactive wnt proteins. Methods Mol Biol 468:17–29. doi: 10.1007/978-1-59745-249-6_2
E Mihara, et al., Active and water-soluble form of lipidated Wnt protein is maintained by a serum glycoprotein afamin/α-albumin., eLife 5 (2016)
