Published by M. Mamunur Rahman, PhD on May 18, 2017 10:04:00 AM
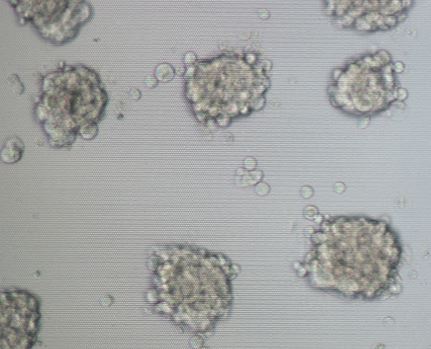
3D cell culture innovation ensures that time spent at the bench will correlate to in vivo environments, with possibilities to aid in future discoveries such as drug delivery into tumor cells or understanding tissue microenvironment. Typically, a researcher use a conventional 2D cell culture technique but are limited to what observations they can truly make. NanoCulture Plate 3D platform has been designed to facilitate researchers’ time in the lab. Two-dimensional (2D) cell cultures are essential for cell biology and has provided vast information to researchers for many years. However, the limitations of 2D cultures are widely recognized, and an improved technique is needed. In recent years, three-dimensional (3D) cell culture has acquired substantial attention due to its potential to deliver higher quantity cell culture information that is representative of true tissue morphology and closely mimics in vivo cell microenvironment (Chiba et al., 2014).
Two-dimensional (2D) cell cultures are essential for cell biology and has provided vast information to researchers for many years. However, the limitations of 2D cultures are widely recognized, and an improved technique is needed. In recent years, three-dimensional (3D) cell culture has acquired substantial attention due to its potential to deliver higher quantity cell culture information that is representative of true tissue morphology and closely mimics in vivo cell microenvironment (Chiba et al., 2014).
The most common 3D culture includes multicellular clusters (spheroids) that are driven by cell‐cell and cell‐matrix contacts, rather than cell‐plastic interactions. Basically, there are two ways to accomplish this: (i) growing the cells in a scaffold and (ii) scaffold free, forcing cells to grow in suspension (Rimann & Graf‐Hausner, 2012).
There are different kinds of gels (animal derived or synthetic) available for scaffold based 3D cell culture and different kinds of methods (i.e. low attachment, U bottom or hanging drop) available for suspension-based 3D cell culture. Though it is argued that scaffold-based 3D culture systems are superior to suspension-based 3D culture system, these methods require troublesome preparation. One such difficulty is that as the culture medium gelates, various problems arise when developing an assay method for drug screening. These problems include affecting drug dispersion, difficulty in harvesting, difficulty in imaging and focusing as the spheroids lie scattered towards the z-axis. Moreover, gels containing biological substances vary from batch to batch, and thus cannot be used by pharmaceutical companies for drug screening. On the other hand, cells’ growth rate is often slow in suspension-based 3D cell culture systems because the cells are not attached to any substrate. Furthermore, cells tend to aggregate by gravity or convection, causing uncontrollable spontaneous fusion that leads to variation in sphere growth. The formation of very large spheres may cause unwanted cell death and /or spontaneous differentiation due to low nutrient and oxygen availability (Bauwens et al., 2008; Serra et al., 2012).
Considering the pros and cons among scaffold-based and suspension-based methods, we developed NanoCulture Plate/ Dish (NCP/NCD). NCP is a SBS format micropatterned plate. It is engineered with an evenly patterned nano-scale structure on a special plastic film attached to the bottom of the plate/dish. This pattern mimics the extracellular matrix and allows cells to migrate across the plate and form healthy spheroids. The pattern eliminates the need for biological/gel or matrix, saves time, and increases reproducibility. It is fully compatible with lab equipment including imaging systems and high throughput screening (HTS) systems. The plate is also compatible with drug screening (Matsuda et al., 2011; Imamura et al. 2015; Yoshi et al. 2015), cancer stem cells (Yamada et al. 2015), co-culture assay (Miyake et al., 2016), EMT assay (Arai et al., 2016), hepatotoxicity assay (Aritomi et al., 2014; Nakamura et al., 2011), ADME assay, primary cell culture, stem cell culture and differentiation (Miyagawa et al., 2011; Obara et al., 2016).
The NanoCulture Plate (NCP) was released onto the market in 2006. It is now sold in more than 10 countries and used by over 300 research groups. The plate is easy to handle and ready to use.

Advantages versus Matrigel or Other Gel Matrix Systems
- Reproducibility
- Cost Savings
- No additional exogenous material
- HTS & HCT compatible
Advantages versus Scaffold Free Plate Systems
Scaffold system is essential for mimicking an in vivo environment
- No Gravity force, Natural formation of spheroids
- Minimal attachment prevents aggregation of spheroids
References:
Aritomi, K., Ishitsuka, Y., Tomishima, Y. et al. (2014) Evaluation of three‐dimensional cultured HepG2 cells in a nano culture plate system: an in vitro human model of acetaminophen hepatotoxicity. Journal of Pharmacological Sciences, 124, 218–229.
Bauwens, C. L., Peerani, R., Niebruegge, S. et al. (2008) Control of human embryonic stem cell colony and aggregate size heterogeneity influences differentiation trajectories. Stem Cells, 26, 2300–2310.
Chiba, T., Suzuki, E., Yuki, K. et al. (2014) Disulfiram eradicates tumor‐initiating hepatocellular carcinoma cells in ROS‐p38 MAPK pathway‐dependent and ‐independent manners. Plos One,9, e84807.
Imamura, Y., Mukohara, T, Shimono, Y. et al. (2015) Comparision of 2D- and 3D-culture models as drug-testing platforms in breast cancer. Oncology Reports, 33, 1837-1843.
Miyagawa, Y., Okita, H., Hiroyama, M. et al. (2011) A microfabricated scaffold induces the spheroid formation of human bone marrow‐derived mesenchymal progenitor cells and promotes efficient adipogenic differentiation. Tissue Engineering Part A, 17, 13–21.
Matsuda, Y., Ishiwata, T., Yamahatsu K. et al. (2011) Overexpressed fibroblast growth factor receptor 2 in the invasive front of colorectal cancer: A potential therapeutic target in colorectal cancer. Cancer Letters, 309, 209-219.
Miyake, M., Hori, S., Morizawa, Y. et al. (2016) CXCL1- Mediated interaction of cancer cells with tumor-associated macrophages and cancer-associated fibroblasts promotes tumor progression in human bladder cancer. NeoPlasia, 18, 636-646.
Nakamura, K., Kato, N., Aizawa, K. et al. (2011) Expression of albumin and cytochrome P450 enzymes in HepG2 cells cultured with a nanotechnology‐based culture plate with microfabricated scaffold. Journal of Toxicological Sciences, 36, 625–633.
Obara, C., Tomiyama, K., Takizawa, K. et al. (2016) Characteristics of three-dimensional prospectively isolated mouse bone marrow mesenchymal stem/stromal cell aggregates on nanoculture plates. Cell Tissue Res., DOI 10.1007/s00441-016-2405-y
Rimann, M. & Graf‐Hausner, U. (2012) Synthetic 3D multicellular systems for drug development. Current Opinion in Biotechnology, 23, 803–809.
Serra, M., Brito, C., Correia, C. & Alves, P. M. (2012) Process engineering of human pluripotent stem cells for clinical application. Trends in Biotechnology, 30, 350–359.
Yoshi, Y., Furukawa, T., Aoyama, H. et al. (2015) Regorafenib as a potential adjuvant chemotherapy agent in disseminated small colon cancer: Drug selection outcome of a novel screening system using nanoimprinting 3-dimensional culture with HCT116-RFP cells. Int. Journal of Oncology. DOI: 10.3892/ijo.2016.3361
Yamada, T., Abei, M., Danjoh, I. et al. (2015) Identification of a unique hepatocellular carcinoma line, Li-7, with CD13(+) cancer stem cells hierarchy and population change upon its differentiation during culture and effects of sorafenib. BMC Cancer, 15, 260. DOI 10.1186/s12885-015-1297-7