Published by Pirouz Daftarian, Ph.D. on Sep 18, 2024 9:47:04 AM
Enhanced Signal-to-Noise Ratio as a Primary Advantage of MBLI Tetramers Concerning Biological Interactions with Dextran
In this blog, we will discuss the increasing evidence in the literature and emphasize key considerations when working with MHC multimers containing dextran. Since, dextran has been used as a backbone for the generation of MHC multimers by various investigators. It is beneficial and informative to discuss the impact of nonspecific bindings and immune cell activation on cell staining, immune monitoring, T cell analysis, and downstream outcomes for investigators. Two important features will be addressed:
1) the interaction of cells of immune cell lineages with glucan, the building blocks of dextran
2) the activation of selected immune cells by glucan / dextran.
Dextran is a complex branched glucan (e.g. β-glucan), or polysaccharide, that is derived from glucose. Dextran is a generic term for a family of glucans that are made by the polymerization of the α-d-glucopyranosyl moiety of sucrose. It is also of note that dextran may be of varying lengths, ranging from 3 to 2000 kilodaltons. Dextran is a type of polysaccharide commonly used in research settings for the isolation and purification of lymphocytes, including T cells. Dextran can interact with cell surface receptors, facilitating isolation through techniques like density gradient centrifugation. In fact, historically, Dextran by its interaction with lymphocytes, allows sedimentation that causes erythrocytes to sediment, while allowing neutrophils and PBMCs to remain in suspension with plasma and dextran (1,2).
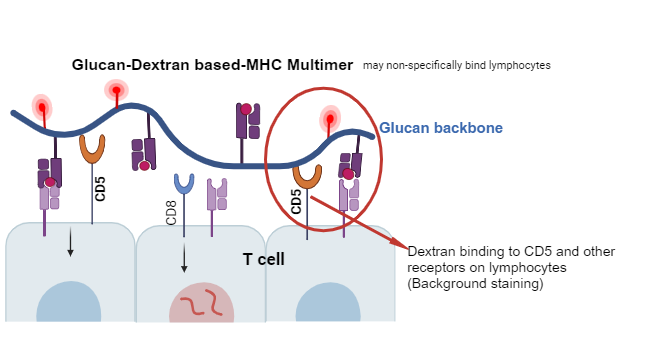
Moreover, dextran via its glucan has the capability to bind and activate cells of innate and adaptive immunity (1-12). The specific surface receptors that have been currently identified for β-glucan receptors are, dendritic cell-associated C-type lectin-1 (Dectin-1), complement receptor 3 (CR3), cluster of differentiation 11b (CD11b)/CD18, αMβ2-integrin, macrophage differentiation antigen-1 (Mac-1), lactosylceramide (LacCer), and scavenger receptors (SRs) (5), and CD5 (1). T cells and B-1 cells express CD5, a scavenger receptor family member that is associated with antigen receptors. A few recent studies have characterized the binding of β-glucan to CD5 on T cells and B-1 cells (1). The affinity of CD5 and β-glucan is calculated as 3.7 × 10−9 M (1). As shown in the figure, dextran, through its beta-glucan, recognizes and stimulates immune responses (2).
In addition, dextran can potentially activate lymphocytes, particularly T cells, through its interaction with antigen-presenting cells (APCs) and the major histocompatibility complex (MHC) molecules (1). During immunization, dextran can be used as a carrier for antigens, where it helps enhance the immune response by promoting antigen presentation to T cells. However, the specific immunological effects of dextran can vary depending on factors such as its molecular weight, and degree of branching (13). In ex vivo settings, dextran sulfate is a trick that immunologists have been using to stimulate mononuclear cells and to elicit the “blast” condition (4).
In summary, the dextran composed of β-glucans has the capacity to interact and activate both adaptive and innate immune response. This type of activation can offer therapeutic applications or present a challenge for researchers. When working with dextran’s back bone MHC multimers, there is an increased risk of false positive activation and misleading results in flow cytometry staining of antigen specific T cells.
The main concern is enhanced background staining and nonspecific bindings, especially in cases where
1) the target T cell clonotype is of low frequency such as neoantigen specific T cells,
2) during sorting practices for single cell analysis.
This potential challenge for researchers can be effectively addressed with a focus on specificity.


MBLI ‘s T cells detection tools focus on identifying specific MHC/peptide complexes by incorporating a patented mutation of the α3 domain in their HLA class I tetramers. MBLI is the only manufacturer of MHC multimers with this advanced technology to enhance specificity for the antigen-specific T cell receptors.
References:
1. Goodridge HS, Wolf AJ, Underhill DM. Beta-glucan recognition by the innate immune system. Immunol Rev. 2009 Jul;230(1):38-50.
2. Cognigni, Valeria, et al. "Potential benefit of β-glucans as adjuvant therapy in immuno-oncology: A review." Exploration of Targeted Anti-tumor Therapy 2.2 (2021): 122.
3. Pelizon A.C., Kaneno R., Soares A., Meira D.A., Sartori A. Immunomodulatory activities associated with β-glucan derived from Saccharomyces cerevisiae. Physiol. Res. 2005;54:557–564.
4. Wang Z, La Rosa C, Maas R, Ly H, Brewer J, Mekhoubad S, Daftarian P, Longmate J, Britt WJ, Diamond DJ. Recombinant modified vaccinia virus Ankara expressing a soluble form of glycoprotein B causes durable immunity and neutralizing antibodies against multiple strains of human cytomegalovirus. J Virol. 2004 Apr;78(8):3965-76.
5. Zhong X, Wang G, Li F, Fang S, Zhou S, Ishiwata A, Tonevitsky AG, Shkurnikov M, Cai H, Ding F. Immunomodulatory Effect and Biological Significance of β-Glucans. Pharmaceutics. 2023 May 29;15(6):1615.
6. Zhong X, Wang G, Li F, Fang S, Zhou S, Ishiwata A, Tonevitsky AG, Shkurnikov M, Cai H, Ding F. Immunomodulatory Effect and Biological Significance of β-Glucans. Pharmaceutics. 2023 May 29;15(6):1615.
7. Chan, G.CF., Chan, W.K. & Sze, D.MY. The effects of β-glucan on human immune and cancer cells. J Hematol Oncol 2, 25 (2009).
8. Pelizon AC, Kaneno R, Soares AM, Meira DA, Sartori A. Immunomodulatory activities associated with beta-glucan derived from Saccharomyces cerevisiae. Physiol Res. 2005;54(5):557-64.
9. Valeria Cognigni, et al. Potential benefit of β-glucans as adjuvant therapy in immuno-oncology: a review Explor. Target. Anti Tumor Ther., 2 (2021), pp. 122-138
10. Bose N, Chan AS, Guerrero F, Maristany CM, Qiu X, Walsh RM, Ertelt KE, Jonas AB, Gorden KB, Dudney CM, Wurst LR, Danielson ME, Elmasry N, Magee AS, Patchen ML, Vasilakos JP. Binding of Soluble Yeast β-Glucan to Human Neutrophils and Monocytes is Complement-Dependent. Front Immunol. 2013 Aug 12;4:230.
11. Chan, G.CF., Chan, W.K. & Sze, D.MY. The effects of β-glucan on human immune and cancer cells. J Hematol Oncol 2, 25 (2009). “β-glucans in fact can directly bind to specific receptors of immune cells”.
12. Ding J, Feng T, Ning Y, Li W, Wu Q, Qian K, Wang Y, Qi C. β-Glucan enhances cytotoxic T lymphocyte responses by activation of human monocyte-derived dendritic cells via the PI3K/AKT pathway. Hum Immunol. 2015 Mar;76(2-3):146-54.
13. Soeiro VC, Melo KR, Alves MG, Medeiros MJ, Grilo ML, Almeida-Lima J, Pontes DL, Costa LS, Rocha HA. Dextran: Influence of Molecular Weight in Antioxidant Properties and Immunomodulatory Potential. Int J Mol Sci. 2016 Aug 19;17(8):1340.